Background: Measurable residual disease assessed by multiparametric flow-cytometry (MFC-MRD) is largely used in trials for childhood de novo acute myeloid leukemia (AML) in the early phase of treatment, to stratify patients into different risk groups and to tailor treatment. Despite this, unlike acute lymphoblastic leukemia, the impact of MFC-MRD before allogeneic hematopoietic stem cell transplantation (HSCT) on patients' outcomes has yet to be established. The aim of this study was to evaluate the prognostic role of MFC-MRD before HSCT in a cohort of children with AML treated in centers belonging to Associazione Italiana Ematologia/Oncologia Pediatrica (AIEOP) cooperative group.
Patients and Methods: We analyzed MFC-MRD in 208 pediatric patients affected by de novo AML who underwent a first allo-HSCT after a myeloablative conditioning regimen in an AIEOP Centre between 12/2011 and 12/2021. Assessment of MFC-MRD was performed on BM sample within 60 days from the transplantation date. Analysis of 5-10 color-panel MFC-MRD was centrally performed at the Laboratory of Diagnosis and Research, University of Padova, according to standardized operating procedures previously described (Buldini et al., BJH 2017). We defined MFC-MRD positivity as the presence of at least 50 leukemic events of 500,000 acquired nucleated cells. Five-year overall (OS) and event-free survival (EFS) were estimated using Kaplan and Meier method and differences between groups were tested using the log-tank test; the cumulative incidence of relapse (CIR) and non-relapse mortality (NRM) were calculated using the Fine&Gray method to take into account the respective competitive risks.
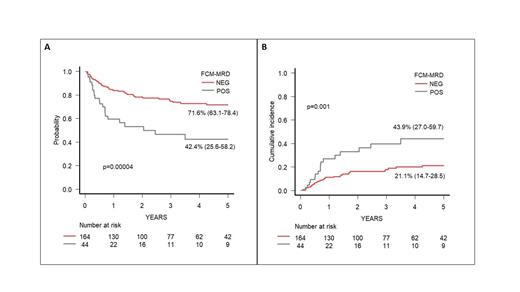
Results: Median age at HSCT of these 208 patients was 7.8 years (range 0.4-23.3); 168 patients (80.8%) were transplanted in CR1, 30 (14.4%) in CR2 and 10 (4.8%) in CR3 or more advanced disease. Forty-eight patients (23.1%) were transplanted from a matched family donor, 85 (40.9%) from an unrelated donor (59 fully-matched and 26 mismatched), while 75 (36%) from a partially matched family donor. The source of stem cells was bone marrow in 117 patients (56.2%), peripheral blood in 81 (39%) and cord blood in 10 (4.8%). Median time from MRD evaluation and HSCT was 22 days (range, 6-57). Overall,168 out of 208 (78.9%) patients were MFC-MRD negative in the pre-HSCT BM, while 44/208 (21.1%) showed a positive MFC-MRD. Among those, MFC-MRD was < 0.1% in 12/44 (27%) patients, 0.1-<1% in 16 patients (36.5%) and ≥ 1% in 16 patients (36.5%). The 5-year OS and EFS of the whole cohort were 79.2% and 65.7%, respectively, while CIR and NRM were 24.7% and 8.6%. Notably, patients with negative MFC-MRD had a significantly better 5-yr OS and EFS (Fig.A) as compared to those with any MFC-MRD positivity (OS 86.3% vs 50.2%; EFS 71.6% vs 42.4%, p <0.0001 in both analysis). CIR significantly differed between patients with MFC-MRD negative and positive (21.1% vs 43.9%, p=0.001) (Fig.B). We also observed a higher NRM in patients with MFC-MRD positivity (13.6%) as compared to those with negative MFC-MRD (7.2%), although this was not statistically significant (p=0.1).
Disease status (CR1 vs CR2 vs other CR) did also influence EFS (69.9% vs 55.6% vs 23.3%, p<0.001), OS (83.2% vs 66.6% vs 40.6%, p=0.02) and CIR (21.1% vs 37.7% vs 66.6%, p<0.001), while other variables (including year of HSCT, type of donor and stem cell source employed) did not. In a multivariable model for OS, EFS and CIR, including MFC-MRD, disease status and year of HSCT, MFC-MRD (pos vs neg) remained statistically significant (HR for OS 3.5, p=0.0004; HR for EFS 2.38, p=0.003; HR for CIR 2.14, p=0.02). In addition, advanced disease phase was an independent risk factor for CIR (HR 1.81, p=0.008).
Conclusion: These data document the independent prognostic value of MFC-MRD before HSCT in a large cohort of pediatric AML patients, using a centralized evaluation by 5-10 color MFC. Patients with negative MFC-MRD before HSCT have a significantly lower risk of treatment failure. In light of these results, we recommend monitoring MFC-MRD before HSCT in pediatric AML patients as a critical tool for therapy-intervention decision before or after HSCT (e.g., additional treatment for improving leukemia control or less intensive/shorter GvHD prophylaxis) with the aim to improve patients' outcomes.
Disclosures
Merli:MEDAC: Speakers Bureau; JAZZ: Consultancy, Honoraria; Amgen: Speakers Bureau; SOBI: Consultancy. Fagioli:Eusa Pharma: Consultancy; Astellas: Speakers Bureau; Bluebird: Speakers Bureau; Medac Pharma: Consultancy, Speakers Bureau; Novartis: Consultancy; Amgen: Consultancy, Speakers Bureau; Bayer: Consultancy; Takeda: Consultancy; Iqone (Clinigen): Consultancy; Gilead: Consultancy. Algeri:Vertex Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Locatelli:Sanofi, Vertex: Membership on an entity's Board of Directors or advisory committees; Miltenyi, Jazz Pharm, Medac, Sobi, Gilead, BluebirdBio: Speakers Bureau; Bellicum, Amgen, Neovii, Novartis. Sanofi, SOBI, Vertex: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal